-40%
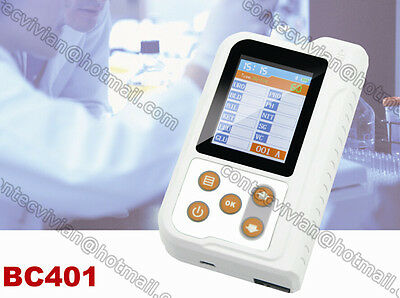
FDA Contec BC401BT Bluetooth Urine Analyzer 11 Parameters 100PCS Test Strips USA
$ 68.11
- Description
- Size Guide
Description
BC401BT Bluetooth Urine Analyzer 11 Parameters 100PCS Test StripsCONTEC MEDICAL SYSTEMS CO.,LTD
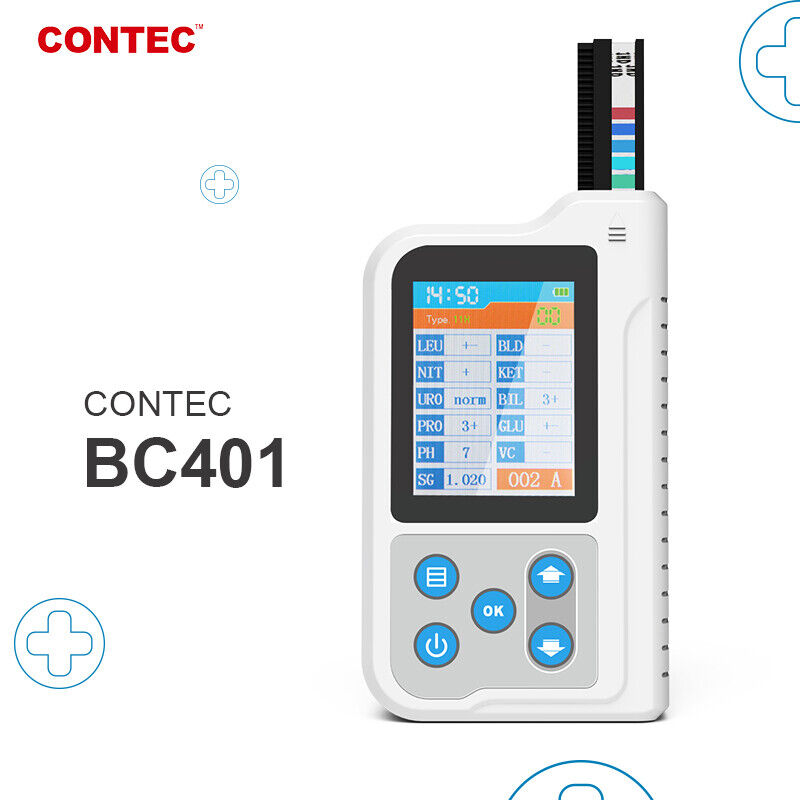
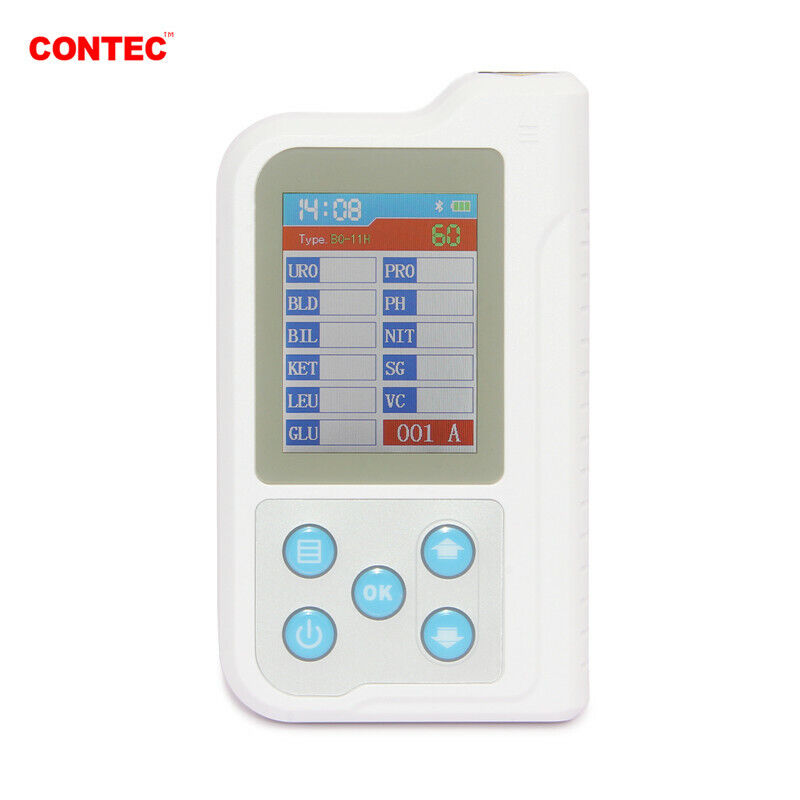
BC401 Urine Analyzer is a high-precision, intellectual instrument which is researched and developed basing on modern optics, electronics, computer science and other advanced technologies for clinical inspection of urine. GLU, BIL, SG, KET, BLD, PRO, URO, NIT, LEU, VC, PH, MAL, CR and UCA in urine can be tested by using it with special test strips. And it is applicable for use in hospital, community health service, clinic, epidemic station and family, etc.
Features
1)High-luminance and white LED, features in long life and good stability.
2)Display abundant contents by the 2.4'' LCD, optional languages: Chinese and English.
3)User-friendly interface.
4)Optional units: international unit, conventional unit and symbol system.
5)Monitoring the whole test process, auto-character and audible prompt.
6)Be compatible with 8, 10, 11, 12, 14-parameter test strip (optional based on the type of test strip).
7)Standard MicroUSB interface, Bluetooth interface (Optional).
Performance
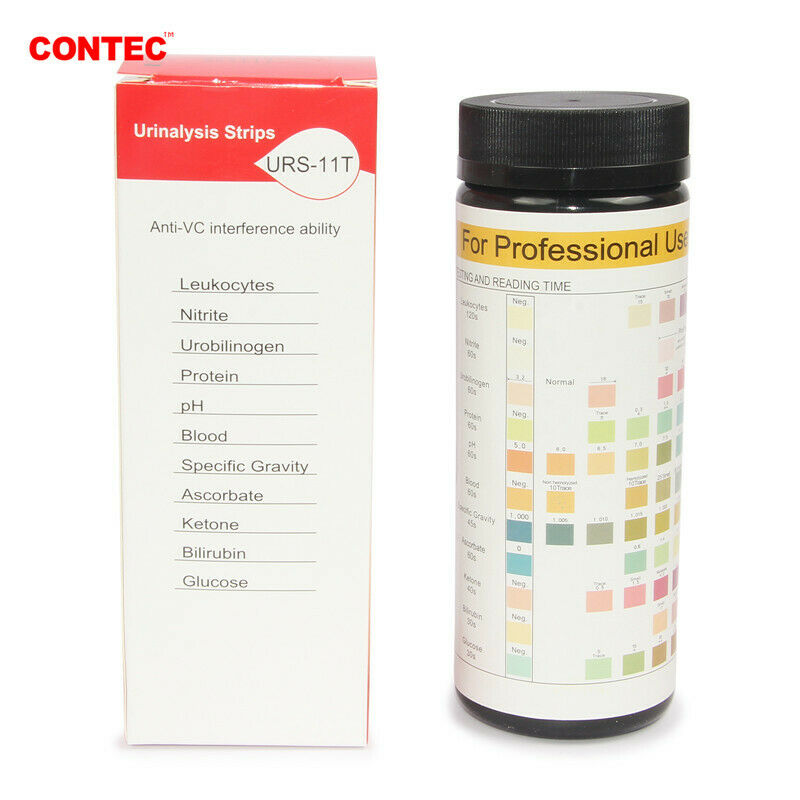
1)Test items: GLU, BIL, SG, KET, BLD, PRO, URO, NIT, LEU, VC, PH, MAL, CR, UCA (optional based on the type of test strip).
2)Test principle: RGB tricolor
3)Repeatability: CV≤1%
4)Stability: CV≤1%
5)Display: 2.4" color LCD
6)Working mode: one-step
7)Test speed: ≥60 tests/hour
8)Data storage: Storage of 500 sample data, which can be queried by test date, sample No. and user name.
9)Interface: Standard MicroUSB interface, Bluetooth interface (Optional).
10)Power supply: DC5V, 1A, built-in rechargeable lithium battery
Accessories
1)Power adapter
2)USB cable
3)User Manual
4)Test strip(100PCS)
Physical characteristic
Dimension: 126mm(L)×73.5mm(W)×30mm(H)
Weight: about 0.18Kg
Working environment:
Temperature: 10 ℃ ~ 30 ℃
Relative humidity: ≤80%
Atmospheric pressure: 76kPa~106kPa
Specified EMC, climate and mechanical environment description: don’t use the device in environment with direct sunlight, the front of open window, flammable and explosive gases, near the heating or cooling equipment, near strong light-source, otherwise it will influence normal use of the device.
Storage environment:
Temperature: -40℃ ~ 55 ℃
Relative humidity: ≤95 %
Atmospheric pressure: 76kPa~106kPa
Specified EMC, climate and mechanical environment description: the packed device should be stored in room with no corrosive gases and good ventilation. Temperature: -40°C~+55°C, relative humidity: ≤95%, and avoid severe impact, vibration, rain and snow during transportation.
Shipping
Clearance: we will ship your item to your Ebay confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
All items will be shipped within 2 business days by E-package,Airmail shipping.
According to shipping method,items will be delivered within 7-35 business days.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved.
About Us
Contec Medical Systems Co.,Ltd; 20 Years manufacturer,we have stock in USA and China.
Contact Us
Please contact us through eBay Message, we are on line to reply your message within 24 hours.
Whatsapp :86-13223300633